Abstract
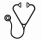
Diffuse large B-cell lymphoma (DLBCL), is one of the most common lymphoma subtypes worldwide, and represents 30% of non-Hodgkin lymphoma (NHL). The current DLBCL chemotherapy R-CHOP regimen is limited by lack of specificity and toxicity to normal hematopoietic cells. Identification of a targeted therapeutic approach is an unmet need for the disease. Through translocations, amplifications or overexpression, c-Myc is dysregulated in many human cancers, including DLBCL. Aberrant overexpression of c-Myc promotes tumorigenesis by activating target genes critical for cell proliferation/survival. In our preliminary study, c-Myc protein levels in 119 cases of newly diagnosed DLBCL were evaluated by immunohistochemistry. We found that c-Myc levels were evident (>30% positive cells) in 40.52% (47/116) and high (>80% positive cells) in 6.90% (8/116) of patients. We also observed that relative to c-Myc negative cases, c-Myc positive cases (>30% positive cells) were associated with adverse prognostic indicators including high international prognostic index (IPI) score (≥60 years old group) (p=0.033), high Ki-67 index (p=0.002), high central nervous system involvement (p=0.003) and low complete response rate (p=0.028), confirming that c-Myc plays a critical role in DLBCL pathogenesis.
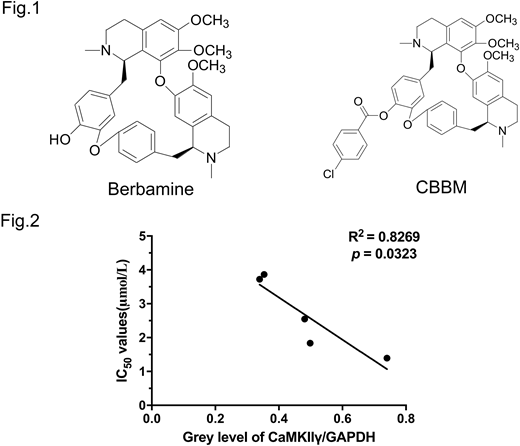
Currently, c-Myc is considered to be an "undrugable" oncoprotein. Chemotherapeutic agents do not affect c-Myc activity. Our previous work in T-cell lymphoma (Cancer Cell, 32:115-128) demonstrated that berbamine, a natural bis-benzylisoquinoline alkaloid, effectively reduced N-Methyl-N-Nitrosourea (MNU)-induced lymphoma burden by disrupting the Ca 2+ /calmodulin-dependent protein kinase II (CaMKII)γ/c-Myc axis. We also showed that CaMKIIγ stabilizes c-Myc by phosphorylating it at the serine 62 residue (Ser62). We created the berbamine derivative 4-chlorobenzoyl berbamine (CBBM) (Fig.1), and evaluated its efficacy in vitro using DLBCL cell lines (n=5) which expressed high levels of c-Myc. The IC50 of CBBM was an order of magnitude lower than that of berbamine across a panel of DLBCL cell lines: the IC50 (μmol/L) of CBBM vs. berbamine in these cells were OCI-Ly3 (1.39±0.04 vs 12.20±0.71), OCI-Ly10 (2.56±0.04 vs 23.66±0.53), U2932 (3.89±0.07 vs 17.78±0.29), SU-DHL 16 (1.82±0.05 vs 17.56±0.71) and Pfeiffer (3.73±0.06 vs 26.16±0.56). CBBM also induced apoptosis in a dose-dependent manner in these cell lines while sparing normal hematopoietic cells from healthy donors (n=2). As an example, CBBM treatment dose-dependently induced apoptosis on OCI-Ly3 cells (vehicle control was 4.43±0.06%, 4 μmol/L CBBM was 24.43±0.72% and 6 μmol/L CBBM was 75.43±0.25%, vehicle control vs CBBM at 4 μmol/L, p<0.001). Conversely, more than 85% of normal hematopoietic cells from mobilized healthy donor peripheral blood were still viable after exposure of CBBM at 10 μmol/L, which is a lethal dose for DLBCL lines. Moreover, CBBM treatment significantly reduced cycling of DLBCL cells (SG2M fractions: vehicle control 68.11±0.40%, CBBM 4 μmol/L 38.36±0.34%, p<0.001). Mechanistically, exposure of DLBCL lines (n=5) to CBBM decreased c-Myc protein levels in a dose-dependent manner. We also validated the reduction of PD-L1 expression, a reported c-Myc target through immunoblots. The proteasome inhibitor MG132 prevented CBBM-induced c-Myc protein degradation, indicating that reduced c-Myc after CBBM treatment is related to downregulation of protein stability. Importantly, we also explored the effect of CBBM on the CaMKIIγ/c-Myc axis. CBBM treatment reduced phospho-c-Myc (Ser62) levels in all the lines. We observed CBBM-mediated inhibitory effects (IC50 of five lines) was correlated with the basal level of CaMKIIγ (R2 = -0.8269, p=0.0323) (Fig.2). Beyond targeting c-Myc, CBBM also exhibited immune modulator potency including downregulation of autocrine interleukin 10 (IL10) level assayed by ELISA, leading to reduction of the downstream JAK2/STAT3 levels. Currently, we are in the process of testing CBBM in vivo effects using an OCI-Ly3 xenograft model. Although the overall survival result is pending, following 4 weeks of CBBM treatment (1 g/kg, i.g.), tumors from animals receiving drug treatment show decreased c-Myc expression levels relative to that of vehicle treated animals. Collectively, our results support further evaluation of CBBM as a promising compound to treat c-Myc associated DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract